Arrange The Following Elements From Greatest To Least Tendency To Accept An Electron Ca Ge K Se Br. It will increase this way and from left to right. Arrange the elements in order of decreasing first ionization energy.
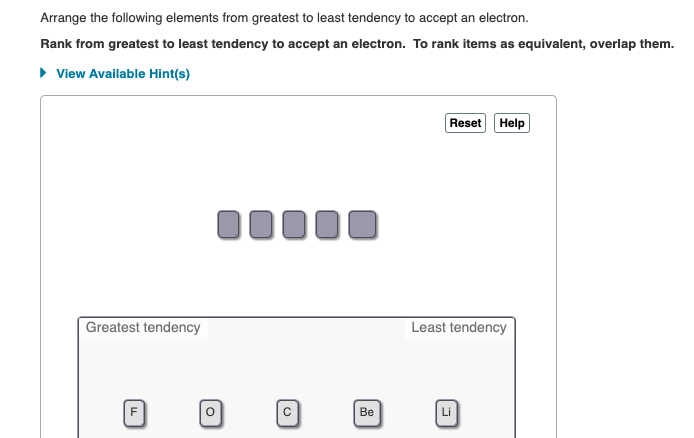
Arrange the following aspects from biggest to least propensity to accept an electronrank from best to least propensity to expropriate an electron. 3 🔴 on a question arrange the following elements from greatest to least tendency to accept an electron. Arrange the following elements from greatest to least tendency to accept an electron.
Arrange The Following Elements From Greatest To Least Tendency To Accept An Electron.
Ordering these elements by the electron affinity provides an. Rank from greatest to least tendency to accept an electron. Be, c, li, o, f *my answer was f, o, c, be, li and i got it , you =)
The Highest Electron Ant Is Bromoma (Br) Belonging To The.
I put them in the above order but, it was wrong it said there are certain exceptions to the periodic trends but, i don't understand what the exceptions are or what the correct order would be Rank from greatest to least tendency to accept an electron. Sr sn rb te i.
Arrange The Following Elements From Greatest To Least Tendency To Accept An Electron.
And that's because so does the nuclear charge the effect of nuclear charge. Arrange the following elements from greatest to least tendency to accept an electron. To rank items as equivalent, overlap them answer:
Arrange The Following Elements From Greatest To Least Tendency To Accept An Electron.?
Br se ge ca k. To rank items as equivalent, overlap them. Rank from greatest to least tendency to accept an electron.
The Elements Usually React If They Have Tendency To Gain Or Lose More Electrons.
Or if they are equivalent overlap them. Arrange the following elements from greatest to least tendency to accept an electron. So overall that turned in electron affinity.