Draw The Lewis Structure Of Hcn. To calculate the valence electron of each atom in caf2, look for its periodic group from the periodic table. Steps of drawing lewis structure of hcl.
Count the valence electrons you can use h + c + n =1 + 4 + 5 = 10 step 3. In the structure, duplet for h atom is complete but the octets of both c and n atoms are incomplete. Learn this topic by watching resonance structures concept videos
The Alkaline Earth Metal And Halogen Families, Which Are The Second And 17Th Groups In The Periodic Table, Are Both Made Up Of Calcium And Fluorine Atoms.
It is a liquid that is colorless, highly toxic, and flammable in nature. Put a pair of electrons connecting the side atom with central atom.put remaining electrons on the side atoms.move electrons fron oxyfgen so that both carbon and oxygens get octet state. Put carbon in the center and hydrogen and oxygen on the sides.
A Hydrogen Atom Has Made A Single Bond With Chlorine Atom.
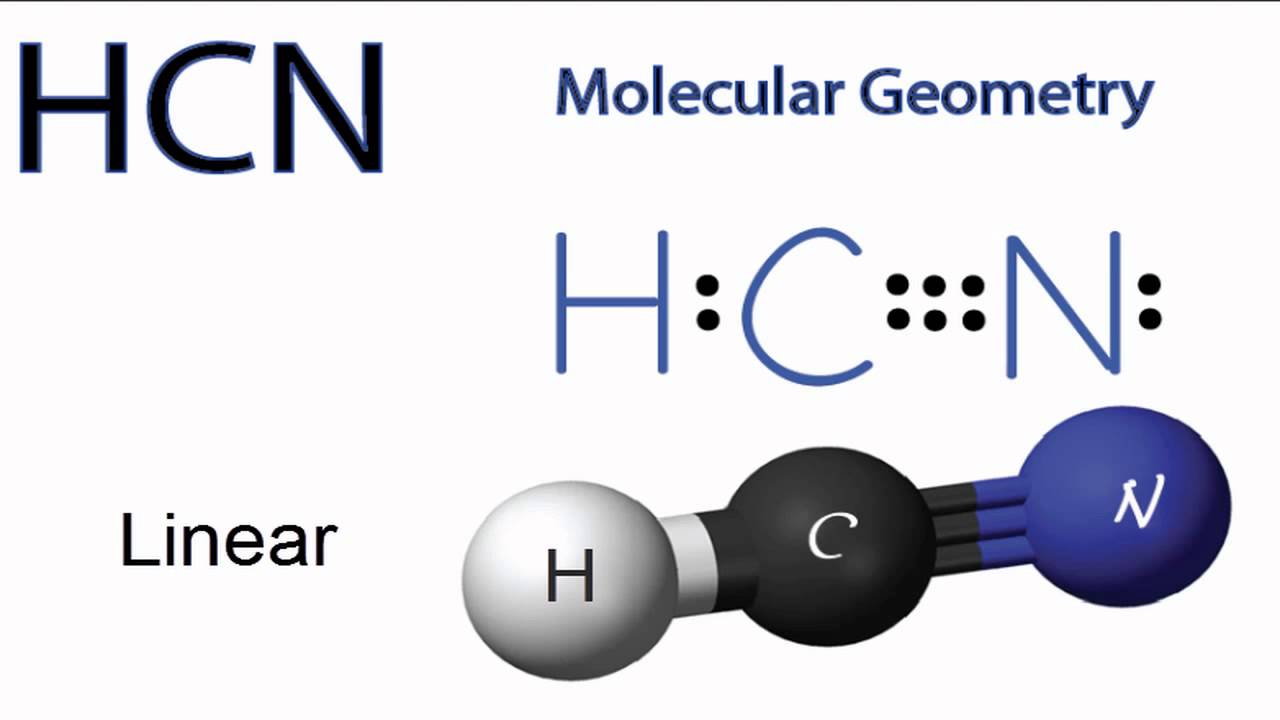
Draw the lewis structure for hcn. It is also called prussic acid. H c n in this example, hcn, the lewis diagram shows carbon at the center with no lone electron pairs.
It Is Written In The Following Steps :
There are no lone pairs on h. There is a lone pair of electrons on n. Ratio of urinary lead excretion to total lead admin was very small 024 009 respectively.
Draw The Lewis Structure & Molecular Geometry For Hcn:
In case of $hcn$ valence electrons are 10 as hydrogen have valency 1, carbon 4 and nitrogen 5. Draw the lewis structure for co32− including any valid resonance structures. There are only one hydrogen atom and one chlorine atom in hcl molecule.
Draw The Lewis Structure Of Hcn.
The number of valence electron is only 1 in hydrogen because it is an exception atom which doesn’t follow the octet rule and thus doesn’t need 8 electrons to fill its octet but needs. We have a total of ten valence electrons for the hcn lewis structure. Lewis dot structure of hcn.