How Many Of The Following Elements Can Form Compounds With An Expanded Octet I O Cl Xe. The mg 2 ⁺ and na ⁺ ion are similar in size as are the s 2 ⁻ and cl ⁻ ions. How many of the following elements can form compounds with an expanded octet?
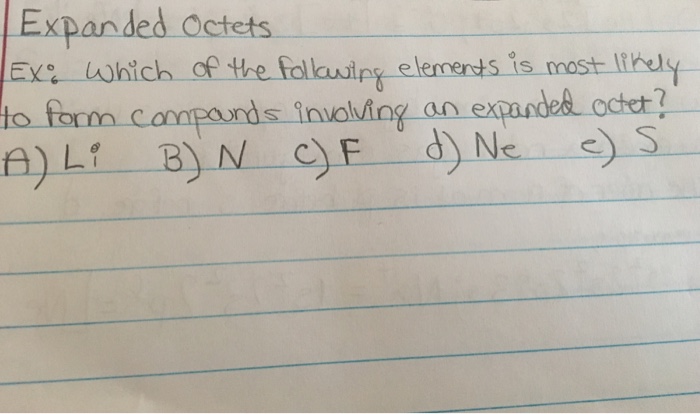
A) 0 b) 1 c) 2 d) 3 e) 4. How many of the following elements can form compounds with an expanded octet?s kr xe ba. P xe as n a) 1 b) 3 d4e) 2 question :
Therefore, The Major Difference In The Lattice Energies For These Two Compounds Is Due To The Difference In The Magnitude Of The Ionic Charge On Each Ion.
Asked sep 20, 2016 in chemistry by yoloko. Which of the following statements is true? So, firstly, the archetypal is where we have eight valence electrons around our central atom in a structure if we're to have an expanded octet than we have more.
P Xe As N A) 1 B) 3 D4E) 2 Question :
4 free expert solution show answer 86% (340 ratings) free expert solution we’re being asked to determine which molecule contains an atom that does not follow the octet rule. How many of the following elements can form compounds with an expanded octet? We’re being asked to determine which of the following elements can form compounds with an expanded octet.
Solution For How Many Of The Following Elements Can Form Compounds With An Expanded Octet?
Exceptions to this rule occur when: Try numerade free for 7 days. How many of the following elements can form compounds with an expanded octet?
A) 0 B) 1 C) 2 D) 3 E) 4.
18) how many of the following elements can form compounds with an expanded octet? Br, n, cl, xe, 0. Exothermic reactions 13 terms hatty_f ruth montag chapter 9 part 1 mastering chemistry 17 terms lazygirlsguidetolife
Here We Will Just Be Identifying Which Of The Following Elements Are Unable To Form Structures With Expanded Octet.
Give the number of valence electrons in $\mathrm{n}, \mathrm{as}, \mathrm{br},$ and se. The mg 2 ⁺ and na ⁺ ion are similar in size as are the s 2 ⁻ and cl ⁻ ions. Which of the following elements can form compounds with an expanded octet.